About Us
CDR Facilities
The CDR is comprised of 26,000 square feet of space designed and equipped specifically for studies of biochemistry, molecular biology and morphology of the Central Nervous System. The area consists of eight laboratories, electron microscope (EM) and confocal microscopy facility, darkrooms, three walk-in cold rooms, four tissue culture facilities, a glassware washing unit, segregated area for radioisotope studies and tissue processing and histology facility.
Bioinformatics
Bioinformatic facility at the CDR is directed by Dr. YunJuan (Janet) Chang.
This site is under construction.
Cytometry and Cell Sorting Facility
The Flow Cytometry and Cell Sorting Facility (Flow) at the CDR, under the direction of Christopher Bare, provides high precision fluorescence probe based analysis and isolation of biological particles including intact viable cells and extracellular vesicles (EV). The Flow facility houses a five laser Invitrogen Bigfoot Cell Sorter capable of discriminating up to 35 separate fluorescent probes on thousands of particles per second, then collecting defined subpopulations of particles for further study in bulk tubes or as individuals in microwells. In addition, Flow staff provide expert knowledge, software training, technology assessment, and project consultation. The highly configurable and adaptable nature of flow allows for a nearly limitless catalogue of applications to obtain data of exceptional granularity. The precision of sorting enables significant target enrichment prior to subsequent exploration in other research modalities. Central to our research is the ability to analyze the various defined populations of intracellular and extracellular compartments from cells in culture, brain tissue, and blood. The instrument allows our researchers to obtain the highest-quality data from every sample to achieve increasingly challenging scientific goals defined within ongoing projects in the CDR and extend those research directions.


Confocal Microscopy Facility
The Center for Dementia Research is equipped with two laser scanning confocal microscopes, an LSM 510 Meta System, and an LSM 880 System, both from Zeiss Microscopy. These systems are used primarily for imaging fixed brain tissue and cell cultures stained with multiple fluorophores. Both systems have heated chambers and CO2 inputs to allow for live imaging of cells in culture with dynamic dyes and can create videos to show changes in real-time. The microscopes have 3 lasers for green red and far-red excitations. The LSM880 can also image blue dyes using a LED and is equipped with the additional capability to make tiled images in a single plane or in z-stacks so that the whole tissue section or cell culture can be reproduced as a 3D rendering. Images can also be analyzed for colocalization between fluorophores or detect relative intensities and sizes of cells and vesicles being studied. CDR confocal microscope analysis has been applied to the human brain and mice modeling Alzheimer’s disease and related disorders (Huntington’s, Parkinson’s, ALS etc.) Cell lines of both human and mouse origins, including fibroblasts from human patients of Familial Alzheimer’s and Down’s syndrome, and primary neurons and blastocysts from mice, are also analyzed in both live imaging and fixed contexts.


Electron Microscopy (EM) Facility
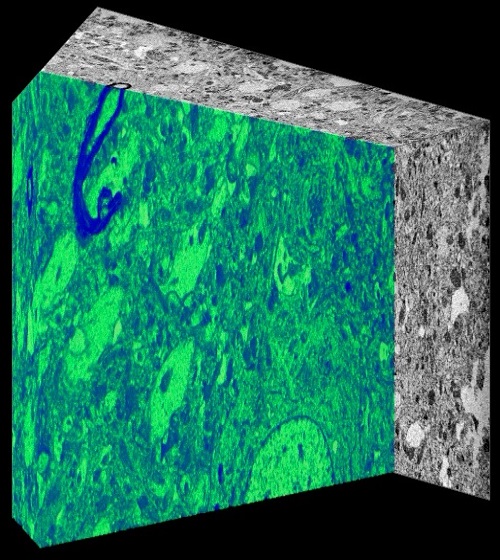
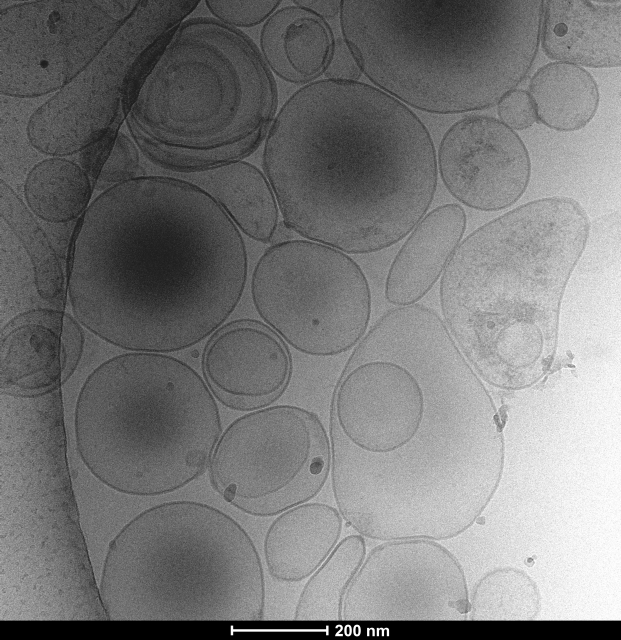
The Electron Microscopy (EM) Facility at the CDR, headed by Dr. Chris Goulbourne is a core facility that provides EM service to investigators. The facility houses a ThermoFisher Talos L120C TEM with 2D imaging capability, dual axis electron tomography for 3D ultrastructural evaluation and cryo EM. Services offered include ultrastructural evaluation in 2D and 3D, protein localization using immunogold techniques and cryogenic evaluation of proteins, viruses, and cellular architecture. Additionally, the facility offers guidance and sample preparation for employing correlative light and electron microcopy (CLEM) and nano secondary ion mass spectrometry (NanoSIMS) for tracking stable isotope tagged molecules. Sample preparation instrumentation includes a Leica UCT ultramicrotome and ThermoFisher Mark IV Vitrobot, as well as a dedicated high-performance workstation for processing 3D data sets using Amira and Imod software. The services of the facility are currently available to external academic institutes and industrial facilities on a fee-for-use-basis. Additional information can be obtained by contacting Dr. Chris Goulbourne at Chris.Goulbourne@NKI.rfmh.org.


Genomics Facility
The Genomics Facility at the CDR, headed by Dr. Stephen Ginsberg enables researchers to conduct a wide variety of genomics paradigms. These include laser capture microdissection (LCM) for downstream applications including quantitative PCR (qPCR) performed at the CDR, as well as RNA sequencing (RNA-seq) and Nanostring nCounter performed by our partners at the New York University Grossman School of Medicine Genome Technology Center. LCM instrumentation in the CDR enables microisolation of desired cell types, including neurons visualized via brightfield or fluorescent optics either through histochemical (e.g., Nissl or hematoxylin and eosin) or immunocytochemical labeling.

Histology Facility
The Histology Facility at the CDR provides researchers with a range of services for histology. These include tissue preparation, embedding, sectioning, histochemical staining, and immunohistochemistry. We offer paraffin, frozen (fixed or fresh), and vibratome sectioning. Tissue sections can also be prepared and processed for downstream DNA, RNA, and/or protein extraction. Sections can be mounted on standard or charged slides for histopathology or molecular and cellular procedures. Frozen sections can be adhered to PEN-membrane slides for laser capture microdissection. Staining options include hematoxylin and eosin, special stains, and immunocytochemical preparations.




